Historic Agreement Opens Door to World’s Largest Food Market—But Only for Those Ready to Meet New Food Safety Standards

On October 26, 2025, Malaysia and the United States signed the Agreement on Reciprocal Trade—a watershed moment that fundamentally transforms market access for Malaysian food and beverage manufacturers. This comprehensive trade pact creates unprecedented export opportunities, but success requires immediate strategic action on food safety compliance and documentation systems.
The time to act is now. While the tariff benefits are substantial, they’re only accessible to manufacturers who can meet stringent US food safety requirements and demonstrate continuous compliance through sophisticated documentation systems.
The Opportunity: Zero-Tariff Access to the $1.5 Trillion US Food Market
The numbers tell a compelling story. Under this agreement, 1,711 Malaysian export products now receive zero-tariff access to the US market. These exempted products are collectively worth USD 5.2 billion and represent 12% of Malaysia’s total exports to the United States.
For Malaysian food manufacturers, the tariff-free list includes critical export sectors:
- Palm oil and palm kernel oil products
- Cocoa products and derivatives
- Rubber-based products
- Pharmaceutical components including Vitamin A and E derived from palm oil
$5.2B
Export Value
Total value of exempted Malaysian products
1,711
Products
Items receiving zero-tariff access
8.1%
Growth Rate
Increase in palm oil exports Jan-Sep 2025
This represents a significant competitive advantage. Malaysia’s palm oil sector alone exported 346,000 metric tonnes to the US from January to September 2025, representing an 8.1% increase over the same period in 2024. The zero-tariff provision effectively lowers the landed cost of Malaysian products, providing distinct commercial advantages against competing nations.
The Compliance Reality: What the Agreement Actually Requires

While the tariff benefits grab headlines, the agreement’s food safety provisions create both challenges and opportunities that Malaysian manufacturers must urgently address.
The path to US market access isn’t just about eliminating tariffs—it’s about demonstrating world-class food safety capabilities through robust documentation, continuous monitoring, and alignment with both Malaysian and American regulatory frameworks.
Facility Recognition
Automatic acceptance of equivalent systems without traditional audits
Electronic Data Exchange
Digital certification and real-time compliance documentation
Harmonized Standards
Dual compliance with Malaysian MS standards and US FDA requirements
Mandatory Recognition of US Food Safety Systems
Article 2.6 of Annex III: The Foundation
Article 2.6 of Annex III requires Malaysia to accept bilateral export certification documents or electronic data elements agreed upon between both governments. This seemingly technical provision has profound implications: Malaysian F&B manufacturers must align their quality assurance systems with US standards to demonstrate equivalence.
The agreement fundamentally transforms the regulatory landscape by requiring Malaysia to recognize US sanitary and phytosanitary (SPS) measures for meat, poultry, and dairy products as meeting Malaysian import requirements. This creates a harmonized regulatory environment where Malaysian exporters must navigate dual compliance frameworks.

Critical Implication: Your quality assurance documentation must now satisfy both Malaysian MS1480/MS1514 standards AND demonstrate equivalence to US FDA requirements. Paper-based systems and fragmented digital records will not suffice for this level of regulatory scrutiny.
Automatic Facility Recognition Without Traditional Audits

Traditional Approach
Facility-by-facility audits, periodic external inspections, reactive compliance posture
New Reality Under Article 2.8
System-based recognition, continuous documentation, proactive compliance demonstration

What This Means for You
Audit-ready documentation 24/7, robust process controls, electronic data accessibility
Article 2.8 mandates that Malaysia automatically recognize US food facilities and inspection systems for dairy, meat, poultry, and aquatic products without imposing additional registration, audit, or inspection requirements.
This system-based recognition approach eliminates traditional facility-by-facility assessments—but creates a reciprocal expectation. Malaysian facilities seeking US market access must demonstrate equivalent capabilities through robust documentation and process control systems. The days of relying solely on periodic external audits are over. Continuous compliance and audit-ready documentation are now business imperatives.
Streamlined Integration: Halal Certification & Residue Limits

Halal Certification Harmonization
Article 2.9 requires Malaysia to accept halal certification issued by US halal certifiers designated by JAKIM “without additional requirements”. For Malaysian manufacturers whose competitive advantage often rests on supplying halal-certified products, this creates opportunities to consolidate halal certification documentation with HACCP, GMP, and US FDA compliance records within unified systems.
Smart manufacturers will integrate halal documentation into their broader food safety management systems rather than maintaining separate, siloed records.

Maximum Residue Limit Alignment
Article 2.13 requires Malaysia to adopt corresponding US maximum residue limits (MRLs) for pesticides and veterinary drugs where Malaysia has not established independent thresholds. This harmonization necessitates sophisticated testing and monitoring capabilities that track residue levels throughout production processes.
Manufacturers must ensure compliance with both Malaysian and US standards simultaneously—a challenge that demands real-time monitoring and automated alert systems when parameters approach critical limits.
Why Manual Compliance Systems Won’t Cut It Anymore
The agreement’s emphasis on electronic data elements, automatic facility recognition, and continuous monitoring creates challenges that traditional paper-based or fragmented digital systems cannot efficiently address.
1. Dual Regulatory Compliance
Malaysian MS1480/MS1514 + US FSMA requirements operating simultaneously across all production processes
2. Real-Time Traceability
Complete visibility from raw materials through finished goods distribution, satisfying FSMA’s Foreign Supplier Verification Program
3. Continuous Audit-Readiness
Documentation systems that provide instant access to compliance records without advance preparation time
4. Electronic Certification
Digital data exchange capabilities compatible with US authority requirements and bilateral agreements
5. Simultaneous Multi-Framework Monitoring
Parallel tracking of HACCP, Halal, FDA, and MS standards within integrated platforms
Industry data reinforces this reality. Facilities implementing IoT-enabled quality monitoring systems report significant improvements in regulatory compliance documentation, with automated systems generating continuous data logs required for FDA inspections.
The auRELIA Solution: Built for This Exact Challenge
The US-Malaysia Agreement on Reciprocal Trade creates precisely the compliance environment that auRELIA’s AI-powered food safety platform was designed to address.
What This Agreement Requires vs. What auRELIA is Designed to Deliver:

Bilateral Export Certification
Agreement: Accept electronic data elements (Article 2.6)
auRELIA: Automated COA generation reducing documentation time by up to 70%
Facility Equivalence
Agreement: Robust process control systems (Article 2.8)
auRELIA: Comprehensive audit trail for every quality control activity

HACCP Integration
Agreement: Unified certification approach (Article 2.9)
auRELIA: Native HACCP documentation alongside GMP, ISO 22000 and Halal

CCP /MRL Monitoring
Agreement: US residue limit alignment (Article 2.13)
auRELIA: Real-time alerts for both Malaysian and US standards
The Platform Malaysian Exporters Can Start Using:

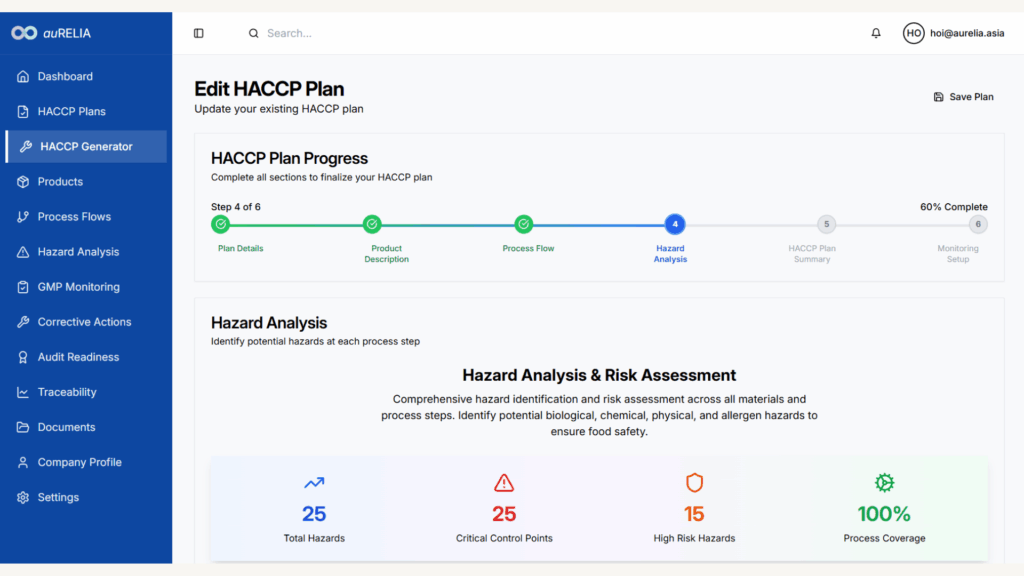
Smart HACCP Plan Generation
Generate comprehensive HACCP Plan Summary aligned with both MS1480 and US FDA requirements in minutes, not days

AI-Generated Process Flow Diagrams
Automated visual workflows that demonstrate process control equivalence to US auditors

Comprehensive Hazard Analysis
AI-powered risk identification applying intelligent validation to detect documentation gaps before audits

Real-Time Monitoring & Traceability
End-to-end visibility from raw material receipt to finished product distribution

Always Audit-Ready Documentation
Continuous compliance posture that eliminates frantic pre-audit preparation
The Competitive Advantage: First-Movers Win
Your Window of Opportunity Is Closing
Indonesia is already moving to match Malaysia’s zero-tariff access, with negotiations targeting palm oil, cocoa, and rubber exemptions. The window for Malaysian manufacturers to capitalize on this first-mover advantage is limited and closing rapidly.
Manufacturers that successfully implement integrated compliance platforms now will be positioned to:
- Secure US supply chain partnerships before competitors build equivalent capabilities
- Demonstrate system-based facility equivalence that unlocks automatic recognition
- Scale export operations without proportionally increasing compliance overhead
- Respond to US buyer due diligence with comprehensive, instantly accessible documentation

Time-Sensitive Reality: US buyers are evaluating Malaysian suppliers right now. Those with sophisticated compliance documentation will secure contracts. Those still relying on manual systems will be left behind as competitors from other nations close the tariff gap.
Your Next Steps: Don’t Leave Money on the Table
The US-Malaysia Agreement on Reciprocal Trade entered into force after a 60-day domestic procedures period. The tariff benefits are real. The market access is unprecedented. But only manufacturers with robust, automated food safety management systems will successfully capitalize on this opportunity
Assess Your Current Compliance Posture
Evaluate whether your existing documentation systems can satisfy dual regulatory requirements and provide instant audit access
Implement Integrated Systems
Deploy automated compliance infrastructure that positions your facility for US market success
Join auRELIA’s Pilot Program
Gain early access to platform features specifically designed for MS1480 + FDA compliance challenges
Demonstrate Export Readiness
Showcase your capabilities to US buyers with comprehensive, instantly accessible documentation
Join auRELIA’s Pilot Program
Position your company at the forefront of intelligent compliance management. As regulatory complexity intensifies globally, early adoption of automated HACCP systems provides strategic advantages that compound over time. Our pilot program offers exclusive access to cutting-edge compliance technology specifically designed for the challenges facing Malaysian exporters in 2025 and beyond.
We’re currently onboarding Malaysian F&B manufacturers to validate our platform’s effectiveness in addressing exactly these dual-compliance challenges. As a pilot program participant, you’ll receive:
- Early access to auRELIA’s MVP 1.0 features specifically designed for MS1480 + FDA compliance
- Direct input into platform development priorities as US market requirements evolve
- Preferential pricing for full platform launch
- Dedicated support from our customer success personnel who understand both Malaysian and US regulatory frameworks
- Case study opportunities to showcase your export readiness to US buyers
Register for our pilot program to experience firsthand how intelligent automation can transform your compliance operations from reactive burden to proactive competitive advantage.
Connect with Our Founders

Ms. Christin Theresa Lim, Co-Founder & CEO
Co-founder of auRELIA Insights, specialising in food safety and regulatory compliance for Southeast Asian markets. Her expertise helps businesses navigate complex international standards and ensure seamless market access.

Mr. Arvindran Salyah, Co-Founder & COO
Co-founder of auRELIA Insights, bringing extensive experience in market access, financial and operational advisory for agri-business, food and beverage companies. He focuses on sustainable growth and compliance efficiency.

auRELIA Insights is backed by Antler and founded by leaders driving the future of work at the intersection of people and technology. Ready to get export-ready faster? Let’s make compliance work for you—not against you
